Lotti Marina
Full Professor of Biochemistry

room 5016, building U3, tel. +39 02 6448 3527
lab 5014, building U3, tel. +39 02 6448 3523
see also: Google Scholar , ORCID, ResearchGate, Linkedin
Graphical abstract
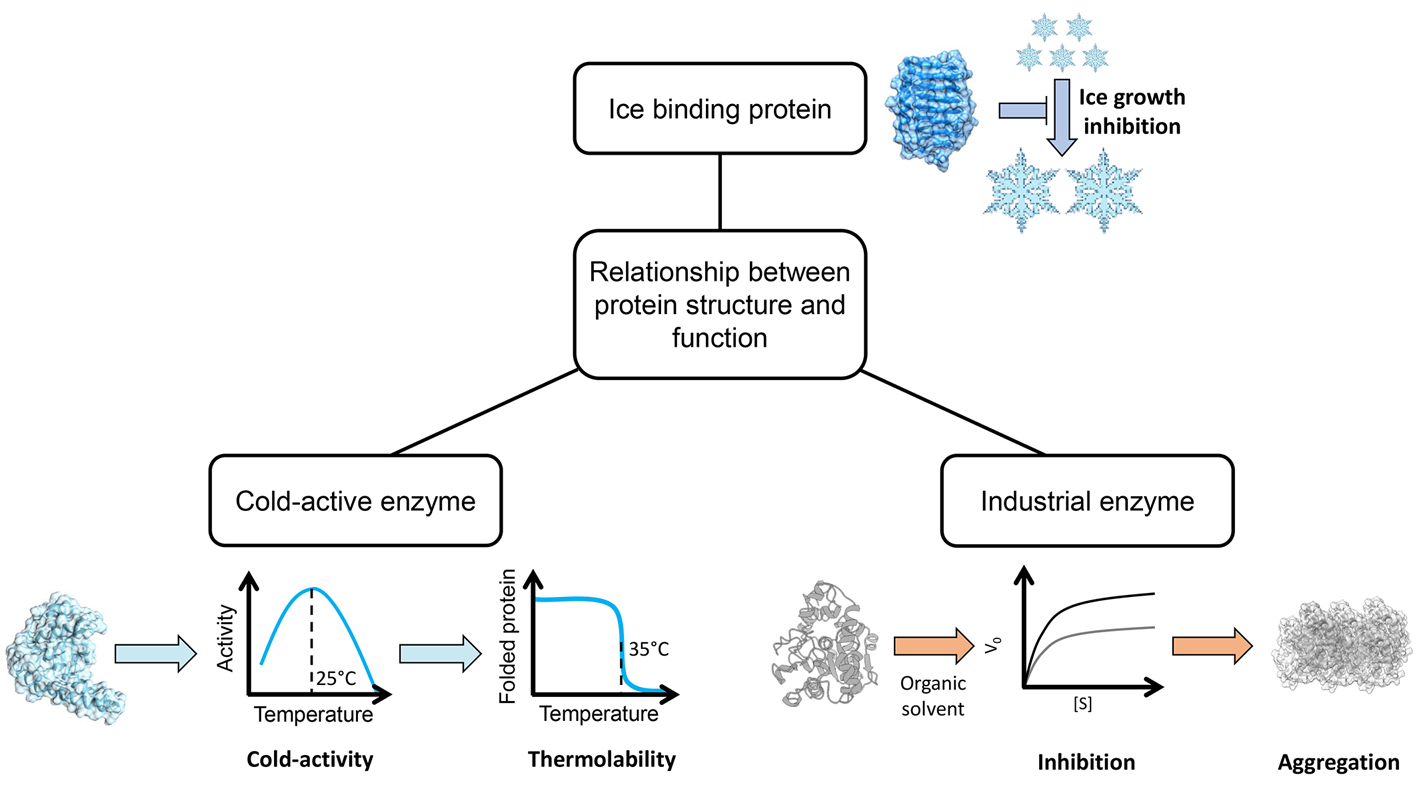
Keywords
Enzymes; Biocatalysis; Protein Engineering; Cold-active enzymes; Antifreeze Proteins
Background
Marina Lotti graduated in Biological Sciences in Milano and got her PhD in Sciences (Naturwissenschaften) at the Max-Planck-Institut fuer Molekulare Genetik in Berlin (Germany) with a thesis of the structure of ribosomes. Presently she is a full Professor of Biochemistry at the University of Milano-Bicocca, where she leads the Laboratory of Protein Engineering and Industrial Enzymology. Currently she serves as a member of the University Board.
Major research topics concern enzymes employed in biocatalysis, and proteins isolated from psychrophilic organisms, in particular enzymes active at low-temperature and ice binding proteins. Another relevant issue is protein folding and misfolding, related to the aggregation of recombinant proteins to form inclusion bodies. These studies employ a combined approach of mutagenesis (both directed evolution and site directed mutagenesis) and biochemical and biophysical characterization.
Research activity is performed in collaboration with several other groups of the Department and with national and international teams.
Teaching is in the frame of the Bachelor and Master degrees in Biotechnology (Biochemistry and Industrial Biochemistry) and of the PhD program in Converging Technologies for Biomolecular Systems. She is one of the directors of the European Summer School of Industrial Biotechnology (http://www.essib.eu/) and one of the organizers of the UNIMIB Summer School “Towards a Bio-based economy: science, innovation, economics, education”. Moreover, ML is involved in the programs of interaction with schools, that are a major goal of the Department.
Active projects
Function and structure of Antarctic glycosydases
We study the features of enzymes from psychrophilic bacteria and lower eukaryotes, identified from genomic and metagenomic analysis. We studied previously superoxide dismutases and are currently engaged in the characterization and exploitation of several glycosydases. These enzymes show very diverse properties and provide hints about their evolution. From the application point of view, enzymes that perform well in the cold are of interest in any energy saving process and in the food industry.
Ice binding proteins
Ice binding proteins, previously known as antifreeze proteins, are produced by organisms exposed to temperature close or even below the freezing point of water as a protection toward the formation of intracellular ice crystals. IBP find application in cryopreservation and in the development of anti-icing materials.
Biocatalysis
We are active in the design and optimization of biocatalytic processes, even in collaboration with companies. In these studies enzymes are characterized as for robustness and performances under operational conditions.
Finished project
Protein aggregation
In several cases, recombinant proteins aggregate to form inclusion bodies (IBs). The structure of proteins embedded in IBs can be modulated by the production conditions and, whenever a relevant amount of native structure is maintained, activity can be preserved. We have studied the mechanism of aggregation, the structure of aggregated proteins and the effects of aggregation on producing cells.
Selected publications
-Mangiagalli M., Ami D., de Divitiis M., Brocca S., Catelani T., Natalello A.*, Lotti M. * (2022) Short-chain alcohols inactivate an immobilized industrial lipase through two different mechanisms. Biotechnology J 2022;2100712. https://doi.org/10.1002/biot.202100712
-Kodera N. , Noshiro D. , Dora S. , Mori T. , Habchi J. , Blocquel D. , Gruet A. , Dosnon M. , Salladini E. , Bignon C. , Fujioka Y. , Oda T. , Noda N. , Sato M. , Lotti M. , Mizuguchi M., Longhi S., Ando T (2021) Structural and dynamics analysis of intrinsically disordered proteins by high-speed atomic force microscopy. NATURE NANOTECHNOLOGY, 16(2), 181-189. PMID: 33230318, DOI: 10.1038/s41565-020-00798-9
-Mangiagalli M, Lapi M, Maione S, Orlando M, Brocca S, Pesce A, Barbiroli A, Camilloni C, Pucciarelli S, Lotti M*, Nardini M* (2021) The co-existence of cold activity and thermal stability in an Antarctic GH42 β-galactosidase relies on its hexametric quaternary arrangement. FEBS J. 288: 546–565. PMID: 32363751, DOI: 10.1111/febs.15354
-Sacchi S., Lotti M., Branduardi P. (2021) Education for a biobased economy: integrating life and social sciences in flexible short courses accessible from different backgrounds. New BIOTECHNOLOGY 60 72–75. PMID: 33039695. DOI: 10.1016/j.nbt.2020.10.002
International and national collaborations
Lotti’s Lab – #LottiLab_BtBs
last update June 2022